Contract Development & Manufacturing (CDMO)
From Concept to Commercial Scale
Your Diagnostic Partner
The development process begins with feasibility assessment and technology selection. Our team evaluates your target analyte, clinical requirements, and market positioning to recommend optimal assay formats, antibody selection strategies, and conjugation approaches. We can source or develop monoclonal/polyclonal antibodies, optimize nanoparticle conjugation protocols, and design membrane systems for maximum sensitivity and specificity.
CDMO Services Include:
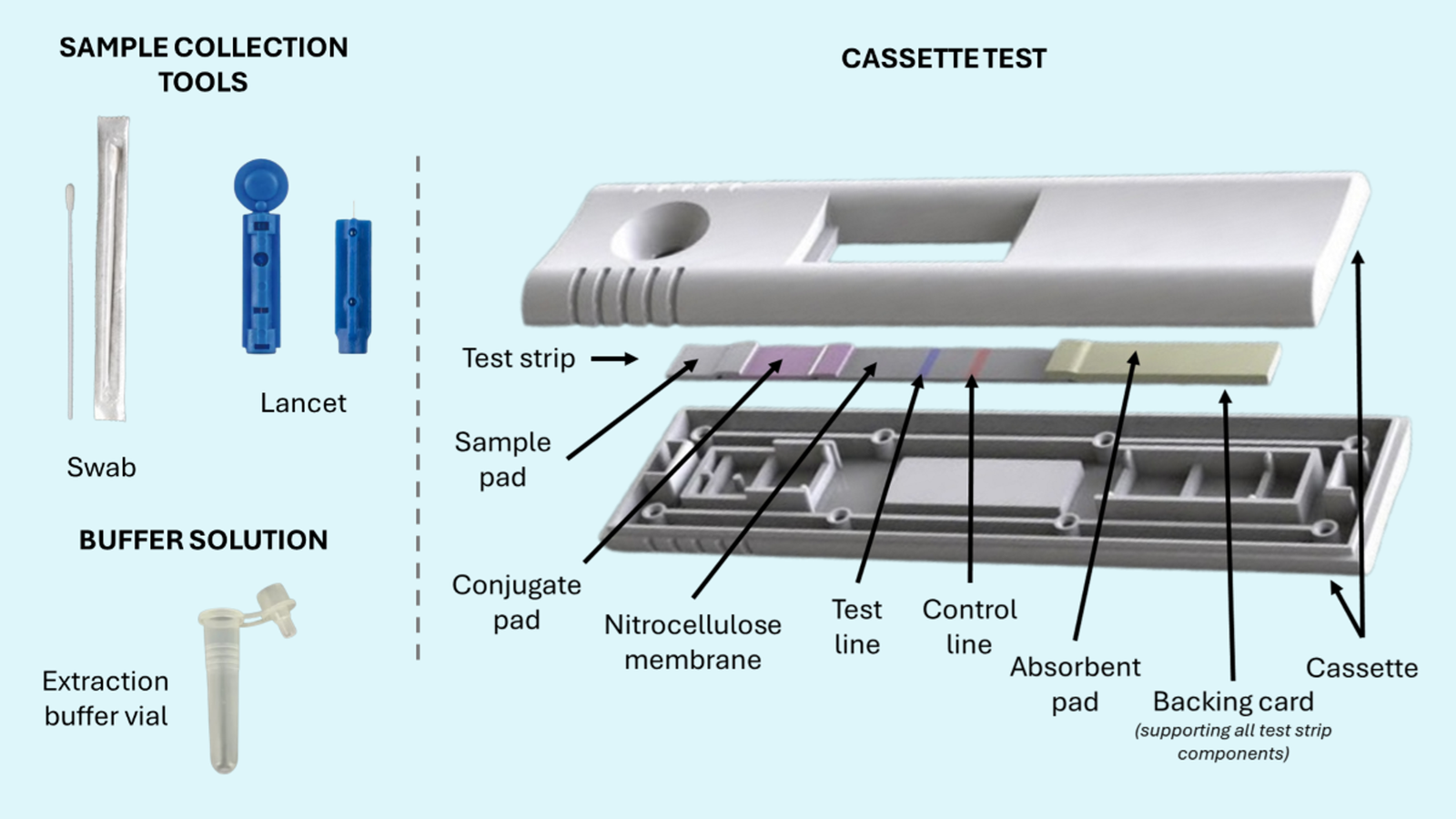
Ideal For
Biotechnology companies without in-house manufacturing capabilities, academic institutions commercializing research discoveries, pharmaceutical companies developing companion diagnostics, public health organizations requiring custom surveillance tools, and medical device companies expanding diagnostic portfolios.
