Clinical Validation & Regulatory Support
Comprehensive Validation Services
Reliability Backed by Data
Retroindica offers end-to-end clinical validation and regulatory support services designed to help diagnostic developers, research institutions, and healthcare organizations navigate the complex pathway from prototype to market-ready product. Our experienced team understands the regulatory requirements across multiple jurisdictions and can design validation studies that meet international standards while addressing specific market needs.
Our analytical validation services include establishment of Limit of Blank (LoB), Limit of Detection (LoD), and Limit of Quantitation (LoQ) through systematic testing protocols. We conduct cross-reactivity assessments against clinically relevant interferents, evaluate assay precision through repeatability and reproducibility studies, and perform stability testing under real-time and accelerated conditions.
Validation Services Include:
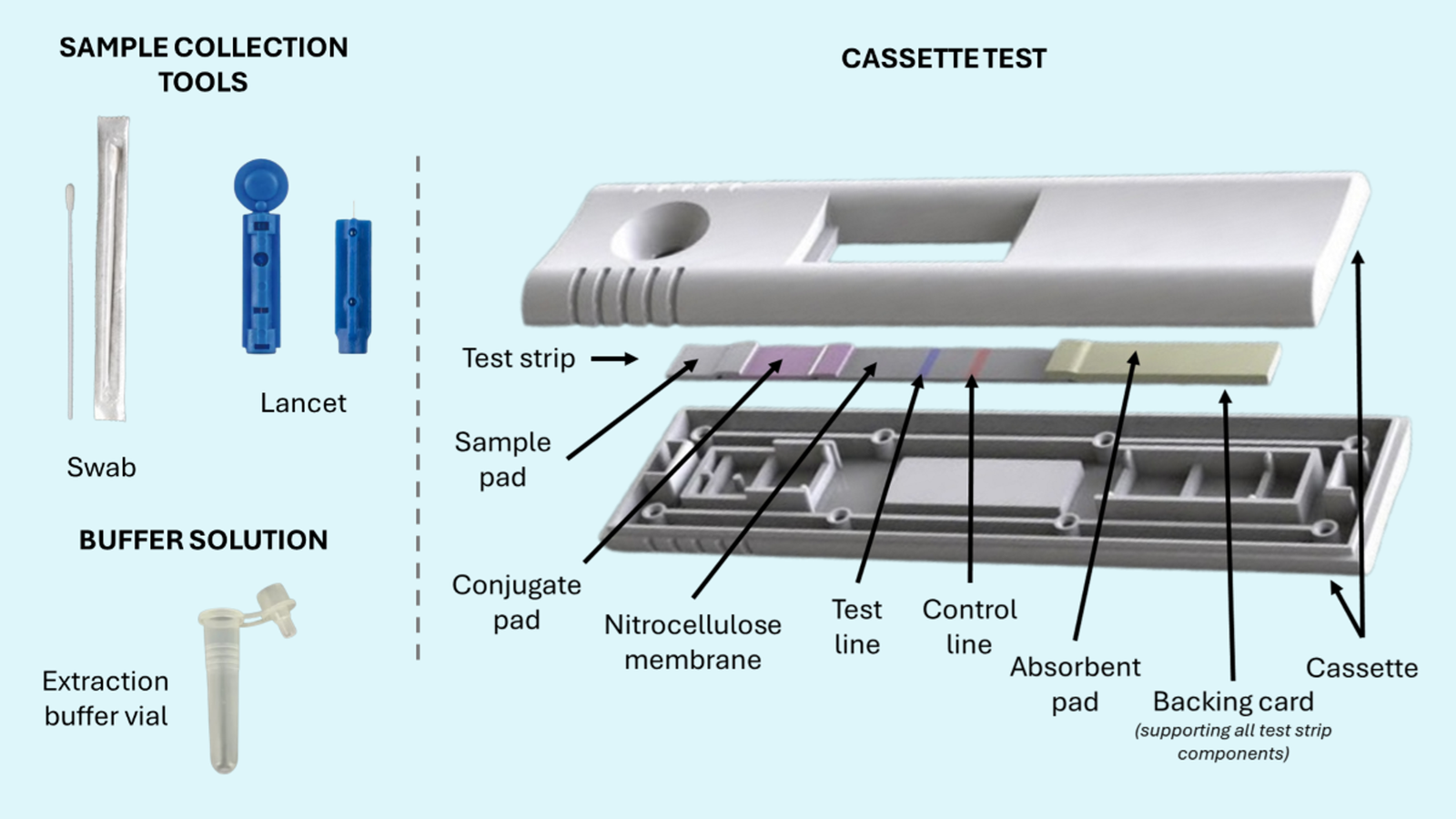
Ideal For
In vitro diagnostic (IVD) developers seeking regulatory clearance, research institutions commercializing novel biomarker assays, healthcare organizations validating in-house diagnostic tests, and companies entering new geographic markets requiring local validation data.
